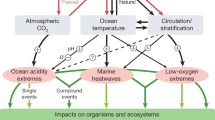
Abstract
Climate change is altering oceanic conditions in a complex manner, and the concurrent amendment of multiple properties will modify environmental stress for primary producers. So far, global modelling studies have focused largely on how alteration of individual properties will affect marine life. Here, we use global modelling simulations in conjunction with rotated factor analysis to express model projections in terms of regional trends in concomitant changes to biologically influential multi-stressors. Factor analysis demonstrates that regionally distinct patterns of complex oceanic change are evident globally. Preliminary regional assessments using published evidence of phytoplankton responses to complex change reveal a wide range of future responses to interactive multi-stressors with <20–300% shifts in phytoplankton physiological rates, and many unexplored potential interactions. In a future ocean, provinces will encounter different permutations of change that will probably alter the dominance of key phytoplankton groups and modify regional productivity, ecosystem structure and biogeochemistry. Consideration of regionally distinct multi-stressor patterns can help guide laboratory and field studies as well as the interpretation of interactive multi-stressors in global models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Doney, S. C. The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516 (2010).
Gruber, N. Warming up, turning sour, losing breath: Ocean biogeochemistry under global change. Phil. Trans. R. Soc. A 369, 1980–1996 (2011).
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Sarmiento, J. L. et al. Response of ocean ecosystems to climate warming. Glob. Biogeochem. Cycles 18, GB3003 (2004).
Riebesell, U. & Tortell, P. in Ocean Acidification (eds Gattuso, J-P. & Hansson, L.) 99–116 (Oxford Univ. Press, 2011).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Bopp, L. et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 10, 6225–6245 (2013).
Hood, R. R. et al. Pelagic functional group modeling: Progress, challenges and prospects. Deep-Sea Res. II 53, 459–512 (2006).
Dutkiewicz, S., Scott, J. R. & Follows, M. J. Winners and losers: Ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycles 27, 463–477 (2013).
Boyd, P. W., Strzepek, R., Fu, F. X. & Hutchins, D. A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 55, 1353–1376 (2010).
Boyd, P. W. & Hutchins, D. A. Understanding the responses of ocean biota to a complex matrix of cumulative anthropogenic change. Mar. Ecol. Prog. Ser. 470, 125–135 (2012).
Edwards, M., Reid, P. C. & Planque, B. Long-term and regional variability of phytoplankton biomass in the Northeast Atlantic (1960–1995). ICES J. Mar. Sci. 58, 39–49 (2001).
Folt, C. L., Chen, C. Y., Moore, M. V. & Burnaford, J. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877 (1999).
Rose, J. M. et al. Synergistic effects of iron and temperature on Antarctic phytoplankton and microzooplankton assemblages. Biogeosciences 6, 3131–3147 (2009).
Hurrell, J. W. et al. The Community Earth System Model: A framework for collaborative research. Bull. Am. Meteorol. Soc. 94, 1339–1360 (2013).
Moore, J. K., Lindsay, K., Doney, S. C., Long, M. C. & Misumi, K. Marine ecosystem dynamics and biogeochemical cycling in the Community Earth System Model CESM1(BGC). J. Clim. 26, 9291–9321 (2013).
Steinacher, M., Joos, F., Frölicher, T. L., Plattner, G-K. & Doney, S. C. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 6, 515–533 (2009).
Boyd, P. W. & Doney, S. C. Modelling regional responses by marine pelagic ecosystems to global climate change. Geophys. Res. Lett. 29, 531–534 (2002).
Moore, C. M. et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nature Geosci. 2, 867–871 (2009).
Boyd, P. W. et al. Marine phytoplankton temperature versus growth responses from polar to tropical waters---Outcome of a scientific community-wide study. PLoS ONE 8, e63091 (2013).
Hutchins, D. A. & Bruland, K. W. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393, 561–564 (1998).
Feng, Y. et al. Effects of increased p CO 2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 388, 13–25 (2009).
Fu, F-X., Warner, M. E., Zhang, Y., Feng, Y. & Hutchins, D. A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J. Phycol. 43, 485–496 (2007).
Balch, W. M. et al. The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Experiment: New evidence in support of the “Great Calcite Belt” hypothesis. J. Geophys. Res. 116 (Special Issue, C00F06), 1–14 (2011).
Johnson, Z. I. et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311, 1737–1740 (2006).
Ward, B. A., Dutkiewicz, S., Moore, C. M. & Follows, M. J. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr. 58, 2059–2075 (2013).
Hutchins, D. A., Fu, F-X., Webb, E. A., Walworth, N. & Tagliabue, A. Taxon-specific response of marine nitrogen fixers to elevated carbon dioxide concentrations. Nature Geosci. 6, 790–795 (2013).
Strzepek, R. F., Hunter, K. A., Frew, R. D., Harrison, P. J. & Boyd, P. W. Iron–light interactions differ in Southern Ocean phytoplankton. Limnol. Oceanogr. 57, 1182–1200 (2012).
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N. & Dunne, J. P. Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167 (2007).
Nielsdóttir, M. C., Moore, C. M., Sanders, R., Hinz, D. J. & Achterberg, E. P. Iron limitation of the postbloom phytoplankton communities in the Iceland Basin. Glob. Biogeochem. Cycles 23, GB3001 (2009).
Zondervan, I. The effects of light, macronutrients, trace metals and CO2 on the production of calcium carbonate and organic carbon in coccolithophores—a review. Deep-Sea Res. II 54, 521–537 (2007).
Tortell, P. D. et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 35, L04605 (2008).
Flombaum, P. et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl Acad. Sci. USA 110, 9824–9829 (2013).
Boyd, P. W. et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 315, 612–617 (2007).
Mulholland, M. R. The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 4, 37–51 (2007).
Hutchins, D. A., Mulholland, M. R. & Fu, F-X. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22, 128–145 (2009).
Thomalla, S. J. et al. Variable export fluxes and efficiencies for calcite, opal, and organic carbon in the Atlantic Ocean: A ballast effect in action? Glob. Biogeochem. Cycles 22, GB1010 (2008).
Geider, R. J., MacIntyre, H. L. & Kana, T. M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 43, 679–694 (1998).
Marinov, I. et al. North–south asymmetry in the modeled phytoplankton response to climate change over the 21st century. Glob. Biogeochem. Cycles 27, 1274–1290 (2013).
Oschlies, A., Schulz, K. G., Riebesell, U. & Schmittner, A. Simulated 21st century’s increase in oceanic suboxia by CO2-enhanced biotic carbon export. Glob. Biogeochem. Cycles 22, GB4008 (2008).
Gehlen, M., Gruber, N., Gangsto, R., Bopp, L. & Oschlies, A. in Ocean Acidification (eds Gattuso, J-P. & Hansson, L.) Ch. 12, (Oxford Univ. Press, 2011).
Moore, J. K., Doney, S. C. & Lindsay, K. Upper ocean ecosystem dynamics and iron cycling in a global 3-D model. Glob. Biogeochem. Cycles 18, GB4028 (2004).
Glover, D. M., Jenkins, W. J. & Doney, S. C. Modeling Methods for Marine Science 571 (Cambridge Univ. Press, 2011).
Conte, M. H., Thompson, A. T., Lesley, D. & Harris, R. P. Genetic and physiological influences on the alkenone/alkenoate versus growth temperature relationship in Emiliania huxleyi and Gephyrocapsa oceanica. Geochim. Cosmochim. Acta 62, 51–68 (1998).
Langer, G. et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 7, Q09006 (2006).
Toseland, A. et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nature Clim. Change 3, 979–984 (2013).
Boyd, P. W., LaRoche, J., Gall, M., Frew, R. & McKay, R. M. L. Role of iron, light, and silicate in controlling algal biomass in subantarctic waters SE of New Zealand. J. Geophys. Res. 104, 13395–13408 (1999).
Martin-Jezequel, V. et al. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 36, 821–840 (2000).
Boyd, P. W. et al. Control of phytoplankton growth by iron supply and irradiance in the subantarctic Southern Ocean: Experimental results from the SAZ Project. J. Geophys. Res. 106, 31573–31583 (2001).
Winter, A., Nendriks, J., Beaufort, L., Rickaby, R. E. M. & Brown, C. W. Poleward expansion of coccolithophores Emiliania huxleyi. J. Plankton Res. 36, 316–325 (2013).
Acknowledgements
S.C.D. acknowledges support from the Center for Microbial Oceanography Research and Education (C-MORE; grant EF-0424599), a National Science Foundation Science and Technology Center. P.W.B. acknowledges support from IMAS, University of Tasmania. S.T.L. acknowledges support from H. Biester and Heinrich-Boell-Foundation.
Author information
Authors and Affiliations
Contributions
P.W.B. and S.C.D. designed the study; S.T.L. carried out the statistical analyses and provided the display items; D.M.G. carried out the statistical analysis; P.W.B. wrote the manuscript with contributions from S.C.D., S.T.L. and D.M.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Boyd, P., Lennartz, S., Glover, D. et al. Biological ramifications of climate-change-mediated oceanic multi-stressors. Nature Clim Change 5, 71–79 (2015). https://doi.org/10.1038/nclimate2441
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate2441
This article is cited by
-
The impact of eddies and spring warm pool on sinking rates of phytoplankton with different shapes and sizes
Marine Biology (2024)
-
Short-term acidification promotes diverse iron acquisition and conservation mechanisms in upwelling-associated phytoplankton
Nature Communications (2023)
-
How climate policy commitments influence energy systems and the economies of US states
Nature Communications (2023)
-
Tipping points of marine phytoplankton to multiple environmental stressors
Nature Climate Change (2022)
-
Swimming performance of sharks and rays under climate change
Reviews in Fish Biology and Fisheries (2022)